Aquyre Biosciences announces that the Van Gogh Microscopy System is now registered and listed with the FDA
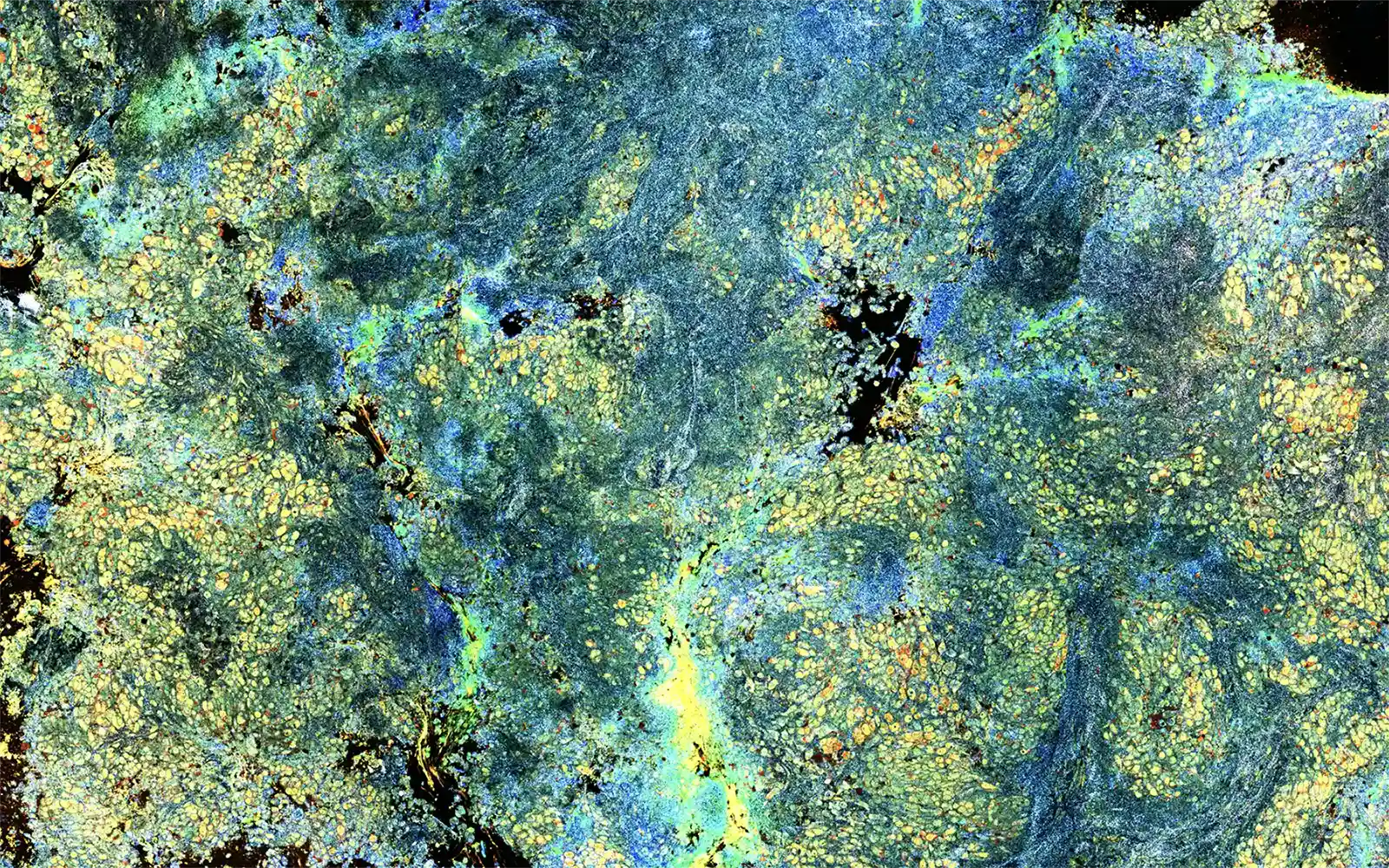
Weston, MA – Aquyre Biosciences, Inc. announces that the Van GoghTM Microscopy System is now registered and listed with the FDA. Van Gogh is a proprietary microscopic imaging system that digitally highlights cells that exhibit elevated metabolic activity.
“This marks an important milestone in our journey to realize the true promise of Rapid Onsite Evaluation (ROSE) through Dynamic Cell Imaging (DCI),” said Greg Bowles, Chief Executive Officer. “It is well known that diseased cells have increased metabolism. The ability to highlight these cells has never been possible before. Our technology is a game-changer in disease diagnosis, drug development and emerging therapeutic treatments.”
From 2021 to 2023, a limited launch took place in the U.S. with a Beta system under the name CelTivity. 900 patients and 4,000 biopsies were imaged with exceptional results, both clinically and technologically.
Today’s announcement marks the beginning of the first steps toward full market release of their biopsy adequacy assessment technology. There is a growing pipeline of customers wanting to purchase systems and engineering has transferred VanGogh’s design to manufacturing after completing its field validation this May.
Contacts
Nicholas Mancini
nmancini@aquyre.com